by Kendrick Fowler
(Kendrick has been a technician with the Program since June 2018 and, as this blog amply illustrates, has ‘gotten into’ wasps.)

Hawthorne Valley Farm in winter.
When I tell someone that I work in entomology, their first reaction is often to ask me something along the lines of “So, what do you do during the winter? ‘Cause there aren’t any bugs around in winter, right?” The answer, of course, is that winter gives me the opportunity to do something with all of the insect specimens that the Farmscape Ecology Program (FEP) team collects during the spring, summer, and autumn (though it is worth pointing out that some very interesting insects can be found crawling around on the snow). Specifically, I identify and count the insects that we captured during the warm months, and I use the resulting data to create content to support the FEP’s research and outreach efforts. Each member of the entomology team, including me, specializes in identifying a few groups of insects whose biology makes them particularly interesting and fruitful subjects on which to focus our studies, usually because they are pollinators, predators, or otherwise considered beneficial to farmers: Conrad works with ground beetles, Dylan has mastered the moths, hover flies, and bees, and I am teaching myself to recognize ants and wasps. I feel like I lucked out when I was assigned my specialty: I am biased, of course, but I feel that the wasps are the most exciting and interesting of our beneficial insects.

My workstation in the Farmscape Ecology Program’s “bug room.” The open boxes on the desk are filled with wasp specimens collected during the 2019 field season.
When most people think of wasps, they think of large, conspicuous, social species like hornets, yellowjackets, and paper wasps. The name “wasp,” however, is also used to encompass an enormous variety of insects that most people rarely (if ever) notice, yet which collectively may have a far larger impact in our lives than the creatures who occasionally invite themselves into our picnics and our homes. Most of these other wasps are small, solitary insects that make their living as what we call parasitoids: organisms that complete their larval development by feeding on or in a single host organism, and that kill their host as a normal part of their development. They are, in essence, real-life versions of the Alien. Fortunately for you and me, they attack only other insects and arachnids, and do not share their fictional analogue’s taste for human flesh.

Wasp cocoons hang from the body of a sphinx moth caterpillar. As larvae, these wasps slowly consumed the caterpillar from the inside. When they were ready to pupate, they worked their way out of the caterpillar’s body and immediately spun cocoons to protect themselves during their transformation into winged adults. A video by The Caterpillar Lab depicting the larvae of wasps similar to these emerging from their host caterpillar is available here (external link).
From the perspective of farmscape ecology, parasitoid wasps are interesting because many species feed on insects that damage crops or that are otherwise regarded as pests, and their activity can therefore contribute to suppressing populations of those insects. It is quite possible—perhaps likely, even—that parasitoids naturally prevent the populations of many kinds of insects from ever reaching levels where they can cause economic injury; it is, however, considerably easier to notice those insects that become pests than those that do not, so any such effect is surely underappreciated. On the other hand, considerable effort has been (and continues to be) expended toward devising ways to manipulate parasitoids into controlling the populations of those insects that do become pests. Often, such work entails introducing parasitoids into regions where they are not native, usually for the purpose of controlling a pest that is likewise an alien in the area; for example, the wasps Tiphia popilliavora and T. vernalis were brought to the eastern United States from Asia during the early 20th century in an attempt to control the Japanese Beetle (Popillia japonica). Another popular strategy involves rearing wasps in large numbers and releasing them at the site of pest infestations to supplement natural causes of mortality (including the activity of wild parasitoids): wasps in the genus Trichogramma, for example, can be purchased by the thousands from commercial insectaries for release into a variety of crops, where they destroy the eggs of caterpillars. Less frequently, attempts are made to increase local populations of wild parasitoids by altering the environment to make it more favorable for their survival and reproduction, such as by planting wildflowers around field edges to provide adult wasps with sources of food. My research into parasitoid wasps at the FEP falls under the latter umbrella: we hope to understand the ecology of our native parasitoids (and other beneficial insects) in order to develop strategies for managing habitats on and around farms to maximize the benefits that these insects provide to agricultural production.

A parasitoid wasp (Microctonus brevipetiolatus) stalks a Striped Flea Beetle (Phyllotreta striolata), a pest of crucifers. M. brevipetiolatus belongs to a group of wasps that share the unusual habit of attacking adult insects; most other species parasitize the immature stages of their hosts.
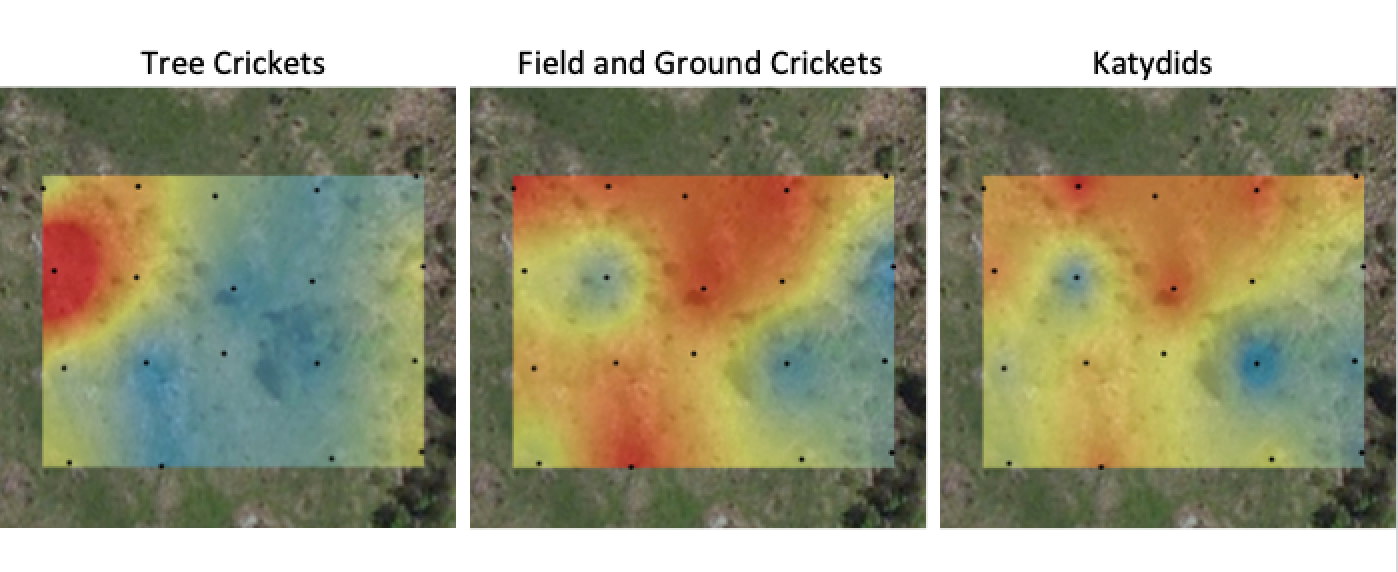
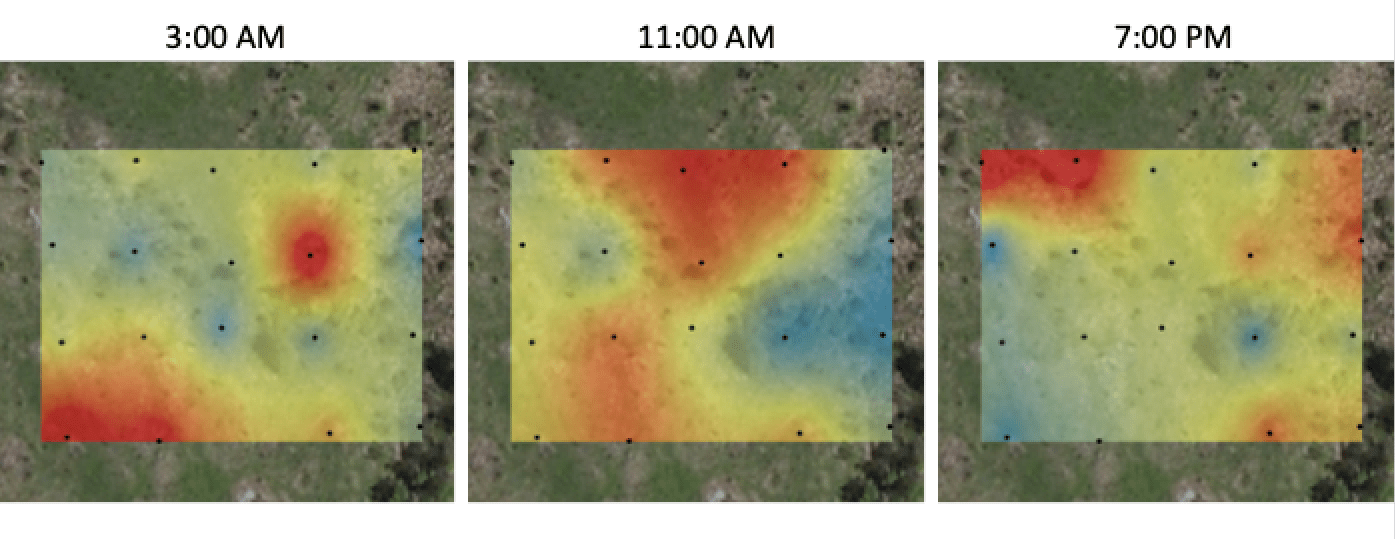
While parasitoid wasps’ usefulness to humans is easily their most popular attribute, I personally find these insects fascinating because they are wonderfully diverse. Over 80,000 species have been discovered worldwide, and they come in a dizzying variety of shapes and colors. Many are decorated in bold, contrasting patterns of black and white, red, orange, or yellow, whilst others resemble tiny jewels, their bodies glimmering with bright, iridescent colors. A few are so miniscule that they approach the physical limits constraining how small an insect can be, and a handful of others grow to impressively large sizes. Even more compellingly, much of the aforementioned diversity is represented here in the Northeast. Some of the largest, smallest, and most colorful wasps in the world are common in our area, and could show up in your backyard or along your local nature trail (assuming, of course, that those places have appropriate habitat). And in terms of numbers, the Northeast is home to many hundreds, if not thousands, of wasp species. During the 2019 field season alone, the FEP collected specimens representing more than 200 species of parasitoid wasps from within a single, 1 mi² study area. Here, I’ll introduce you to just a small selection of my favorites.

Parasitoid wasps of all shapes and sizes collected during the FEP’s 2019 field season. A disk of the same diameter as a penny and an Eastern Yellowjacket (Vespula maculifrons) are included in the image to provide a size comparison. From left to right, the wasps are: Baeus sp., Cheiloneurus sp., Torymus sp., Diadegma stenosomus, Tiphia sp., and the aforementioned yellowjacket. Baeus is so small that it appears as merely a speck! Except for the yellowjacket (which is not a parasitoid), all of these wasps are profiled below.

Bracon sp.
Braconidae: Bracon sp.
The most famous of the parasitoid wasps are those that belong to the closely-related families Braconidae and Ichneumonidae, which together contain many of our largest, most conspicuous, and most economically important parasitoids. The Braconidae take their name from the genus Bracon, some species of which are common and rather pretty. The needle-like structure at the end of the pictured specimen’s abdomen is its ovipositor, the organ that wasps use for laying their eggs. A pair of flexible sheaths cover the ovipositor when it is not in use; here, the near one is bent to reveal the ovipositor nestled inside.

Epimicta konzaensis

The outward facing mandibles of Epimicta konzaensis. (The mandibles have been digitally outlined for clarity.)
Braconidae: Epimicta konzaensis
Braconids in the subfamily Alysiinae share the unusual trait of having mandibles with teeth that curve outward, away from their mouth opening (outlined in the image above). All alysiines parasitize flies, and they use their unusual mandibles to escape from the hardened shelters (called puparia) inside which many flies pupate and to dig through detritus in search of new hosts. One of the most interesting alysiines in our collection is Epimicta konzaensis, a rare species that went undiscovered until the early 2000s, when a handful of individuals were captured in Kansas and Tennessee; our specimens might be the first collected from the Northeast.

Colpotrochia crassipes
Ichneumonidae: Colpotrochia crassipes
The bold yellow-and-black coloration of Colpotrochia crassipes, an ichneumonid, gives it a striking resemblance to a yellowjacket or a potter wasp. Conspicuous color patterns such as the one that these wasps share (often called aposematic or warning colors) are believed to function as a signal to predators that the organisms bearing them are well-equipped to defend themselves when attacked. Ichneumonids and braconids lack the potent stings of yellowjackets (and other related wasps), but they will nevertheless attempt to jab attackers with their ovipositors, and they are also speculated to produce noxious chemicals that make them unappetizing meals. It is worth noting that, despite their sometimes intimidating appearance, all parasitoid wasps are non-aggressive and harmless to humans (although a few particularly large species with short, stout ovipositors might give a painful prick if handled carelessly).

Diadegma stenosomus
Ichneumonidae: Diadegma stenosomus
In my opinion, some of our most elegant wasps are those that belong to the ichneumonid subfamily Campopleginae, like this Diadegma stenosomus. Though widespread in North America, the sole host reported for D. stenosomus in the scientific literature is Carcina quercana, a non-native moth that has only established populations in the Pacific Northwest. In our area, then, D. stenosomus must be parasitizing other moths—presumably, native species related to C. quercana—but their precise identities have yet to be documented.

Diplazon laetatorius
Ichneumonidae: Diplazon laetatorius
Diplazon laetatorius is probably the most widespread ichneumonid in the world: it has been recorded on every continent (except perhaps Antarctica) and on a number of small oceanic islands. It is sometimes regarded as a pest, as it parasitizes the larvae of aphid-eating hover flies, which are some of our most important beneficial insects.

Mesochorus sp.
Ichneumonidae: Mesochorus sp.
Remarkably, some wasps attack other parasitoids, a phenomenon called hyperparasitism. Some hyperparasitoids, including Mesochorus, seek out hosts that are still developing as larvae inside the bodies of other insects, while others, sometimes called pseudohyperparasitoids, wait until their hosts pupate—and thus, in most cases, have emerged from their victims’ bodies—to attack. The Caterpillar Lab has produced an excellent video of a hyperparasitoid wasp (but not Mesochorus) in action; you can watch it here (external link).

Cheiloneurus sp.
Encyrtidae: Cheiloneurus sp.
Some of the most colorful of our parasitoid wasps belong to a large group of closely-related families collectively referred to as the Chalcidoidea. Most of these species, however, are quite tiny, and it is difficult to appreciate their beauty without the aid of a microscope. This Cheiloneurus, for example, is slightly smaller than a mustard seed.

Torymus sp.
Torymidae: Torymus sp.
Many chalcidoids, like this Torymus, are colored in shimmering, metallic shades of green and blue. Though brilliant to our eyes, these colors might function as camouflage, hiding the wasps amongst green vegetation or making them difficult to follow when they move between sun and shade.

Blepyrus sp.
Encyrtidae: Blepyrus sp.
When viewed in the right light, the wings of most parasitoid wasps shine with patterns of bright, rainbow-like colors. Long regarded as little more than a curiosity, it has recently been discovered that each wasp species possesses its own, unique pattern of colored wing reflections, and it is now believed that these patterns function as some kind of communication signal; perhaps wasps flaunt the reflections when interacting with each other. The colors are formed by a process similar to the one that gives a soap bubble its psychedelic sheen; unlike the ever-changing pattern on a soap bubble, however, that of a wasp’s wing remains consistent regardless of the angle from which it is observed.

Paracentrobia sp.
Trichogrammatidae: Paracentrobia sp.
Many of our smallest wasps, such as these Paracentrobia, complete their entire development inside the eggs of other insects. It boggles the mind to imagine a creature small enough to rely on an insect egg as its sole source of food (three of the pictured Paracentrobia could crowd onto the head of a pin)—yet a remarkable video produced by The Caterpillar Lab, available here (external link), shows not one but several wasps sharing a single moth egg. The smaller wasps in the video are probably a relative of Paracentrobia.

Neomymar vierecki
Mymaridae: Neomymar vierecki
Many of the tiniest insects, including many parasitoid wasps, develop wings in which the membrane is largely replaced by a fringe of long bristles. This characteristic is particularly pronounced in wasps belonging to the chalcidoid family Mymaridae (commonly called “fairyflies”), such as this Neomymar vierecki. The adaptive value of bristled wings is not well-understood; however, at the minute scale of these wasps, air’s viscosity has a much greater effect on the way air flows around the wing than it does for larger insects, birds, or airplanes; as a consequence, air flows poorly through the gaps between the bristles, and a bristled wing can exert almost as much force against the air as one formed by a continuous membrane. Meanwhile, it has been suggested that drag caused by friction between the air and the wing surface might be considerably lower in a bristled wing than in a similarly-sized membranous one because the bristled wing has a much smaller surface area. It therefore appears likely that bristled wings are an aerodynamic adaptation that minimizes the effort required for flight in the smallest insects by trading a small decrease in power for a larger reduction in drag.

Pseudometagea schwarzii
Eucharitidae: Pseudometagea schwarzii
One of our strangest chalcidoids, Pseudometagea schwarzii belongs to a family of wasps (Eucharitidae) whose members are all specialized parasitoids of ants. P. schwarzii in particular parasitizes the larvae of the Cornfield Ant (Lasius neoniger). Unlike most parasitoid wasps, P. schwarzii and its relatives lay their eggs on vegetation near ant colonies rather than directly on or in their hosts, and the newly-hatched larvae are free-living. When the larva is passed by a worker ant, it clings to the ant and hitches a ride back to the colony and into the ants’ brood chamber, where it burrows into the body of an ant larva and then—in the case of P. schwarzii—becomes dormant for many months, overwintering inside its host. The wasp begins to feed as the ant larva completes its development in the spring, and it pupates inside its host’s cocoon. Upon emergence, the adult wasps leave the ant nest to mate and start the cycle again.

Inostemma sp.
Platygastridae: Inostemma sp.
The large horn on the back of this Inostemma is a structure for housing the wasp’s ovipositor. Inostemma belongs to a group of wasps (Platygastroidea) that carry the entire length of their ovipositors internally. Those species that require long ovipositors (for attacking hosts concealed in plant tissues, soil, or other hard-to-reach locations) have consequently developed remarkable shapes and structures to create space for the organ inside their tiny bodies. Dorsal humps and horns are particularly common, appearing across a number of unrelated species within the family, but only rarely do their proportions become as spectacularly bizarre as they do in Inostemma.

Baeus sp
Platygastridae: Baeus sp.
Big-headed and squat-bodied, wasps in the genus Baeus are fundamentally cute. These miniscule wasps (many are smaller than a poppy seed) are parasites of spider eggs. The females’ wings are reduced to tiny scales, an adaptation which renders them flightless but might help them to burrow through the silken walls of their hosts’ egg sacs. Most of the world’s Baeus species remain undescribed, even in well-studied regions such as the Northeast. Imagine: an insect that no human has ever documented could be crawling around in your garden!

Tiphia sp.
Tiphiidae: Tiphia sp.
Wasps in the genus Tiphia are some of our most common and conspicuous parasitoids, though their hairy bodies might cause one to mistake them for bees. Tiphia parasitize the larvae of scarab beetles, and as mentioned previously, two Asian species, T. popilliavora and T. vernalis, were introduced to the eastern U.S. during the early 20th century in an attempt to control the Japanese Beetle (Popillia japonica). Many native species also occur in our area.

Insect traps set in the Farmscape Ecology Program’s experimental pollinator meadows. Several distinct habitats appear in this image: a native wildflower meadow, a fallow field, a field planted in rye (used as a cover crop), a riparian woodland, and an upland forest. All are home to a myriad of parasitoid wasps.
Want to see parasitoid wasps for yourself? In the Northeast, any well-vegetated habitat is likely to be home to an abundance of these wasps, and in the warm months finding one can be as simple as going for a walk in the woods and keeping a sharp eye out for insects. Large species, like the spectacular American Pelecinid (Pelecinus polyturator), can be quite conspicuous during the time of year when they are active. However, most parasitoid wasps are quite small and difficult to detect, and will be much easier to find by following some kind of intentional, targeted search strategy. Like many other insects, parasitoid wasps are attracted to nectar and honeydew, and can be found visiting flowers or aggregations of aphids and other plant-sucking insects. Presumably due to their small size, parasitoid wasps seem to prefer to visit small flowers; those of plants in the carrot family, such as Queen Anne’s Lace (Daucus carota), Water Hemlock (Cicuta maculata), and Wild Parsnip (Pastinaca sativa), can be particularly rewarding places to search. Alternatively, if your interest lies in finding wasps that parasitize particular insect hosts, searching locations where those host species occur can likewise be a fruitful strategy. It is also possible to rear wasps by taking potential host insects into captivity and keeping them alive to see whether parasitoids emerge (though one must be careful to avoid collecting host species that are rare or threatened). Many of our parasitoid wasps have not been associated with their hosts, and discoveries are waiting to be made for anyone reasonably dedicated to seeking new rearing records.

The American Pelecinid (Pelecinus polyturator), one of our largest and most conspicuous parasitoid wasps, is a common sight in Northeastern woodlands during July and August. Its long, flexible abdomen allows it to probe deep into soil in search of its hosts, the larvae of June beetles (Phyllophaga spp.).
Bibliography
Resources preceded by an asterisk (*) were freely available online at the time of publication, and are hyperlinked. Most of the others can be acquired at your local public library via interlibrary loan, or directly through the library at your local college or university. Readers interested in a detailed, but accessible general introduction to wasps and their relatives are encouraged to seek out Eric Grissell’s Bees, Wasps, and Ants: The Indispensable Role of Hymenoptera in Gardens, which is available from many college libraries (external link); it also is reasonably affordable should one wish to purchase a copy.
Allen, H.W. 1966. A Revision of the Tiphiinae (Hymenoptera: Tiphiidae) of Eastern North America. Transactions of the American Entomological Society 92(2): 231-356.
Austin, A.D., N.F. Johnson, and M. Dowton. 2005. Systematics, Evolution, and Biology of Scelionid and Platygastrid Wasps. Annual Review of Entomology 50: 553-582.
*Ayre, G.L. 1962. Pseudometagea schwarzii (Ashm.) (Eucharitidae: Hymenoptera), a parasite of Lasius neoniger Emery (Formicidae: Hymenoptera). Canadian Journal of Zoology 40: 157-164. (external link)
*Balaban, J., et. al. BugGuide [Internet]. Ames, IA: Iowa State University. Species Carcina quercana – Oak-skeletonizer Moth – Hodges#1069 [updated 2018 February 15, cited 2020 Jan. 22]. Available from: https://bugguide.net/node/view/166225. (external link)
Barbosa, P. 1998. Conservation Biological Control. Academic Press, San Diego, California, U.S.A.
*Bennett, A.M.R. 2003. Host location behaviour in Pelecinus polyturator (Hymenoptera: Pelecinidae). Journal of the Entomological Society of Ontario 134: 131-134. (external link)
Borror, D.J. and R.E. White. 1970. A Field Guide to the Insects of America North of Mexico. Houghton Mifflin Company, Boston, Massachusetts, U.S.A.
*Buckingham, G.R. and M.J. Sharkey. 1988. Abdominal exocrine glands in Braconidae (Hymenoptera). In V.K. Gupta (ed.) Advances in Parasitic Hymenoptera Research, E.J. Brill, Leiden, South Holland, Netherlands, pp. 199-242. (external link)
*Buffington, M.L. and R.J. Sandler. 2011. The occurrence and phylogenetic implications of wing interference patterns in Cynipoidea (Insecta: Hymenoptera). Invertebrate Systematics 25: 586-597. (external link)
Cheng, X. and M. Sun. 2018. Very small insects use novel wing flapping and drag principle to generate the weight-supporting vertical force. Journal of Fluid Mechanics 855: 646-670.
Cheng, X. and M. Sun. 2019. Revisiting the clap-and-fling mechanism in small wasp Encarsia formosa using quantitative measurements of wing motion. Physics of Fluids 31: 101903.
Crompton, J. 1955. The Hunting Wasp. Houghton Mifflin Company, Boston, Massachusetts, U.S.A.
*Doucet, S.M. and M.G. Meadows. 2009. Iridescence: a functional perspective. J. R. Soc. Interface 6: S115-S132. (external link)
Eggleton, P. and K.J. Gaston. 1990. “Parasitoid” species and assemblages: convenient definitions for misleading compromises?. Oikos 59(3): 417-421.
Gibson, G.A.P., J.T. Huber, and J.B. Woolley, eds. 1997. Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera). NRC Research Press, Ottawa, Ontario, Canada.
Godfray, H.C.J. 1994. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press, Princeton, New Jersey, U.S.A.
*Goulet, H. and J.T. Huber, eds. 1993. Hymenoptera of the world: An identification guide to families. Centre for Land and Biological Resources Research, Ottawa, Ontario, Canada. (external link)
Grissell, E. 2010. Bees, Wasps, and Ants: The Indispensable Role of Hymenoptera in Gardens. Timber Press, Portland, Oregon, U.S.A.
*Heraty, J.M. 1985. A revision of the Nearctic Eucharitinae (Hymenoptera: Chalcidoidea: Eucharitidae). Proceedings of the Entomological Society of Ontario 116: 61-103. (external link)
Hoffman, M.P. and A.C. Frodsham. 1993. Natural Enemies of Vegetable Insect Pests. Cornell Cooperative Extension, Ithaca, New York, U.S.A.
*Huber, J.T. and J.S. Noyes. 2013. A new genus and species of fairyfly, Tinkerbella nana (Hymenoptera, Mymaridae), with comments on its sister genus, Kikiki, and discussion on small size limits in arthropods. Journal of Hymenoptera Research 32: 17-44. (external link)
*Kula, R.R. and G. Zolnerowich. 2005. A new species of Epimicta Förster (Hymenoptera: Braconidae) from North America and new distribution records for Epimicta griffithsi Wharton. Proc. Entomol. Soc. Wash. 107(1): 78-83. (external link)
Marshall, S.A. 2006. Insects: Their Natural History and Diversity: With a photographic guide to insects of eastern North America. Firefly Books, Buffalo, New York, U.S.A.
Marshall, S.A. 2012. Flies: The Natural History and Diversity of Diptera. Firefly Books, Buffalo, New York, U.S.A.
Mason, C.W. 1927. Structural Colors in Insects. II. The Journal of Physical Chemistry 31(3): 321-354.
*Murray, T., B. Moisset, and B. Carlson. BugGuide [Internet]. Ames, IA: Iowa State University. Species Diplazon laetatorius – Hover fly parasite [updated 2012 June 7, cited 2020 Jan. 22]. Available from: https://bugguide.net/node/view/176654. (external link)
O’Neill, K.M. 2001. Solitary Wasps: Behavior and Natural History. Comstock Publishing Associates, Ithaca, New York, U.S.A.
Poulin, R. 2011. The Many Roads to Parasitism: A Tale of Convergence. Advances in Parasitology 74: 1-40.
Pucci, T.M. 2013. Contributions to the classification of North American Microctonus (Braconidae, Euphorinae). Zootaxa 3725(1): 1-150.
Quicke, D.L.J. 2015. The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics, Evolution, and Ecology. John Wiley & Sons, Chichester, West Sussex, U.K.
Ruxton, G.D., T.N. Sherratt, and M.P. Speed. 2004. Avoiding Attack: The evolutionary ecology of crypsis, warning signals, and mimicry. Oxford University Press, Oxford, U.K.
Sane, S.P. 2016. Neurobiology and biomechanics of flight in miniature insects. Current Opinion in Neurobiology 41: 158-166.
*Schwarzfeld, M.D. 2014. Ichneumonidae (Hymenoptera) of the Canadian Prairies Ecozone: A Review. pp. 317-397 In D.J. Giberson and H.A. Cárcamo, (eds.) Arthropods of Canadian Grasslands (Volume 4): Biodiversity and Systematics Part 2. Biological Survey of Canada, Ottawa, Ontario, Canada. (external link)
*Shevtsova et al. 2011. Stable structural color patterns displayed on transparent insect wings. Proceedings of the National Academy of Sciences of the United States of America 108(2): 668-673. (external link)
Smith, O.J. and A. Peterson. 1950. Microctonus vittatae, a Parasite of Adult Flea Beetles, and Observations on Hosts. Journal of Economic Entomology 43(5): 581-585.
*Stevens, N.B. and A.D. Austin. 2007. Systematics, distribution, and biology of the Australian ‘micro-flea’ wasps, Baeus spp. (Hymenoptera: Scelionidae): parasitoids of spider eggs. Zootaxa 1499: 1-45. (external link)
*Tooker, J.S. and L.M. Hanks. Flowering Plant Hosts of Adult Hymenopteran Parasitoids of Central Illinois. Annals of the Entomological Society of America 93(3): 580-588. (external link)
Townes, H. 1971. The Genera of Ichneumonidae, Part 4. The American Entomological Institute, Ann Arbor, Michigan, U.S.A.
*Townes, H. and M. Townes. 1959. Ichneumon-Flies of America North of Mexico: 1. Subfamily Metopiinae. Bulletin of the United States National Museum 216: 1-318. (external link)
*Valerio, 1984. Two alternative strategies for spider egg parasitoids. Revista de Biologia Tropical 32(1): 123-128. (external link)
Wäckers, F.L., P.C.J. van Rijn, and G.E. Heimpel. 2008. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biological Control 45(2): 176-184.
Wahl, D.B. 1993. Cladistics of the genera of Mesochorinae (Hymenoptera: Ichneumonidae). Systematic Entomology 18: 371-387.
Walley, G.S. 1967. Nearctic species of the Diadegma stenosomus complex (Hymenoptera: Ichneumonidae). The Canadian Entomologist 99: 925-943.
*Weems, H.V., Jr. 1954. Natural enemies and insecticides that are detrimental to beneficial Syrphidae. The Ohio Journal of Science 54(1): 45-54. (external link)
*Wharton, R.A., P.M. Marsh, and M.J. Sharkey, eds. 1997. Manual of the New World Genera of the Family Braconidae (Hymenoptera). The International Society of Hymenopterists, Washington, D.C., U.S.A. (external link)
Wylie, H.G. and C. Loan. 1984. Five Nearctic and one introduced Euphorine species (Hymenoptera: Braconidae) that parasitize adults of crucifer-infesting flea beetles (Coleoptera: Chrysomelidae). The Canadian Entomologist 116: 235-246.
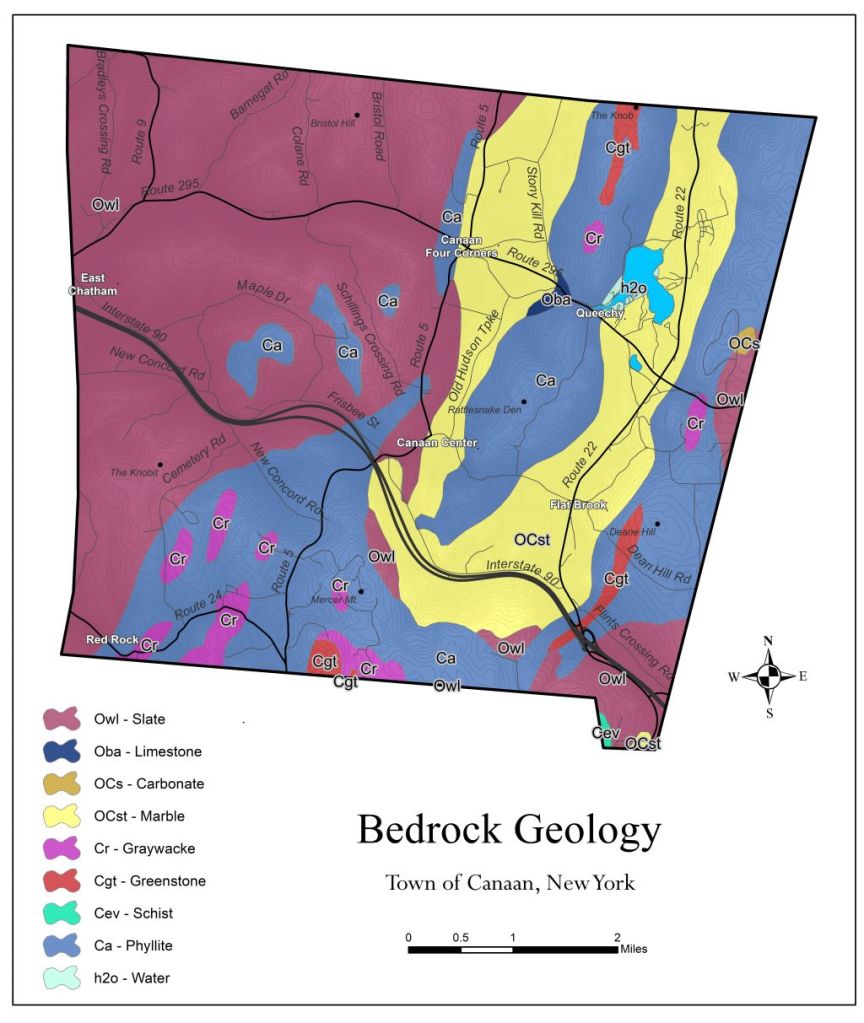





































































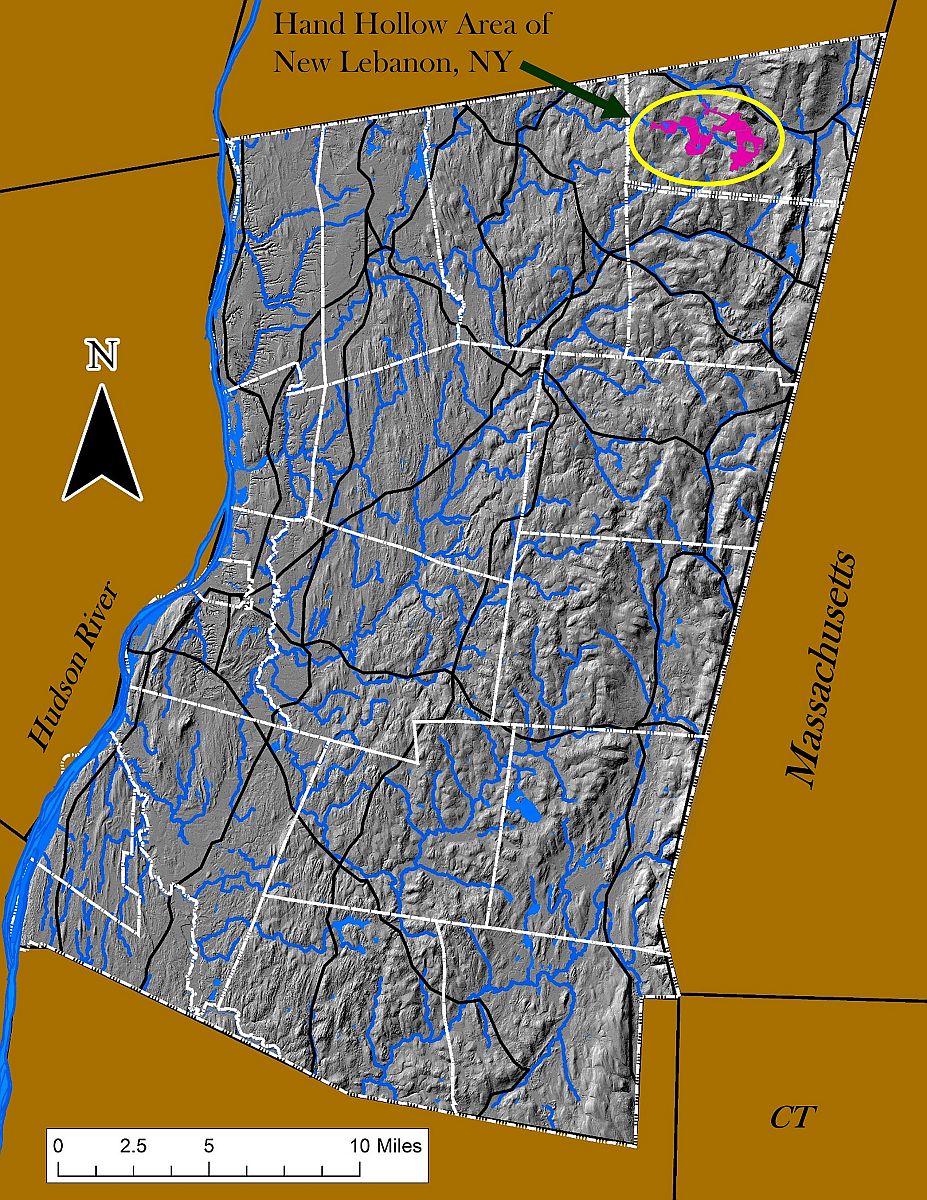
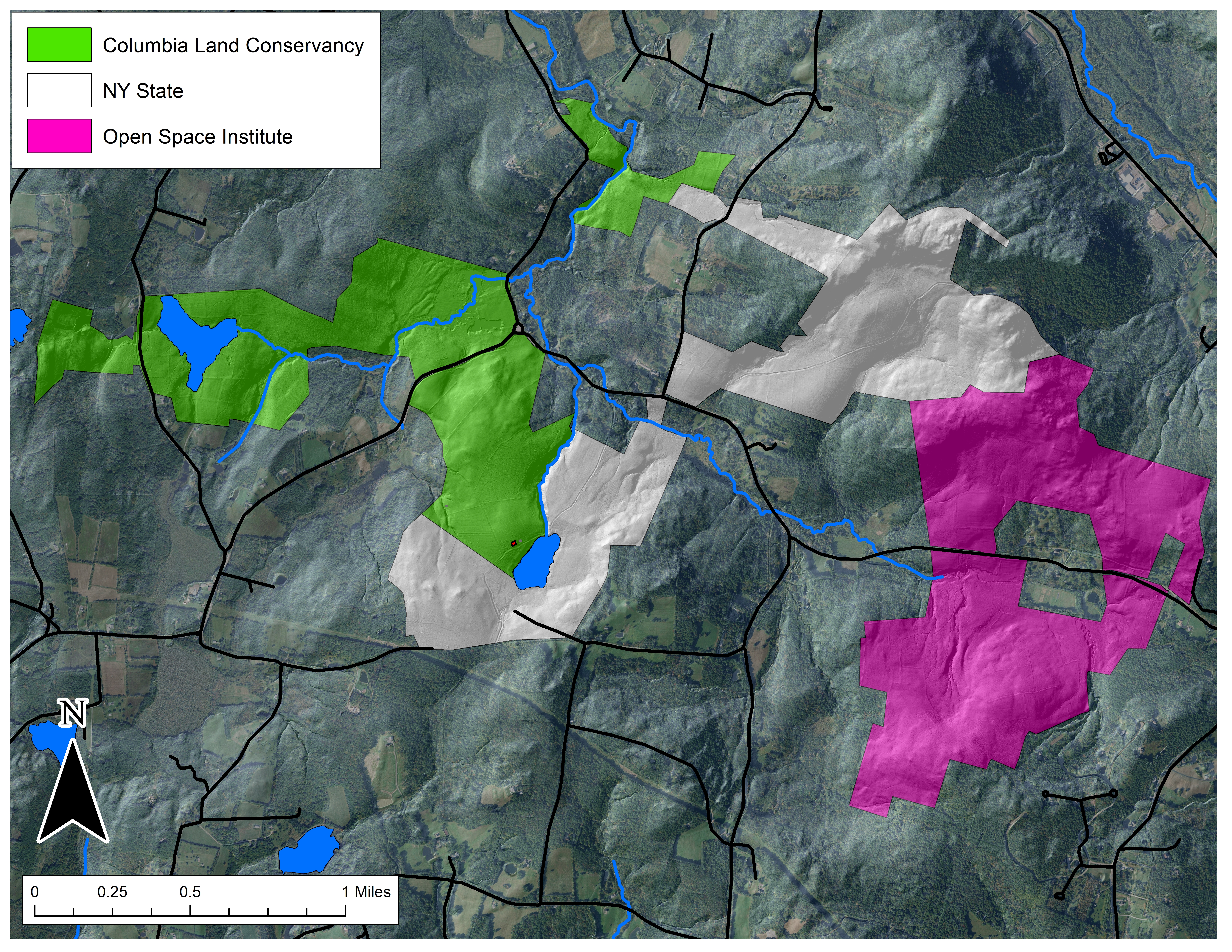 The Hand Hollow State Forest is part of a mosaic of conservation lands in this New Lebanon neighborhood (which was once called New Britain). The Columbia Land Conservancy’s Hand Hollow Conservation Area is to the west, the State Forest itself stretches both north and south of County Route 34, while to the east the Open Space Institute is holding lands earmarked for eventual inclusion in the State Forest.
The Hand Hollow State Forest is part of a mosaic of conservation lands in this New Lebanon neighborhood (which was once called New Britain). The Columbia Land Conservancy’s Hand Hollow Conservation Area is to the west, the State Forest itself stretches both north and south of County Route 34, while to the east the Open Space Institute is holding lands earmarked for eventual inclusion in the State Forest.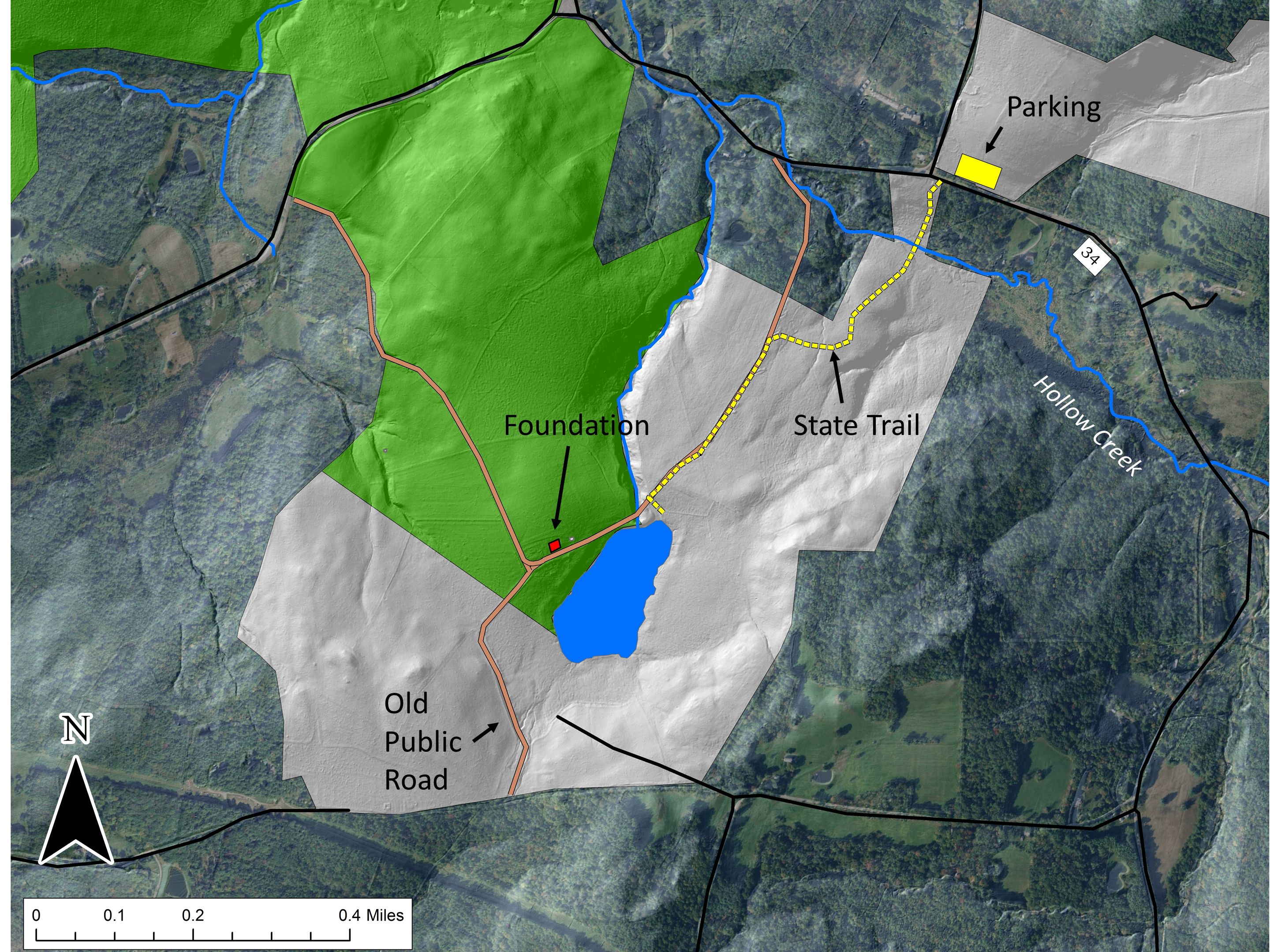 On Saturday last, we walked south from the State Forest parking area north of route 34. The trail weaves its way to the pond, and follows an old public road for its southern half.
On Saturday last, we walked south from the State Forest parking area north of route 34. The trail weaves its way to the pond, and follows an old public road for its southern half. Between route 34 and Hollow Creek is a nice stand of old Hemlock. Judging by size (an admittedly risky criterion) some of these trees may well be over 200 years old. It seems unlikely that this area was ever completely opened, perhaps because it is so wet.
Between route 34 and Hollow Creek is a nice stand of old Hemlock. Judging by size (an admittedly risky criterion) some of these trees may well be over 200 years old. It seems unlikely that this area was ever completely opened, perhaps because it is so wet. A bit farther along, the forest was heavily cut over in the early 2000s, but the rough topography and the presence of Yellow Birch and American Beech suggest that this part too was never completely opened for agriculture.
A bit farther along, the forest was heavily cut over in the early 2000s, but the rough topography and the presence of Yellow Birch and American Beech suggest that this part too was never completely opened for agriculture. We soon hit the old public road, following its deeply worn, rock-bordered way.
We soon hit the old public road, following its deeply worn, rock-bordered way.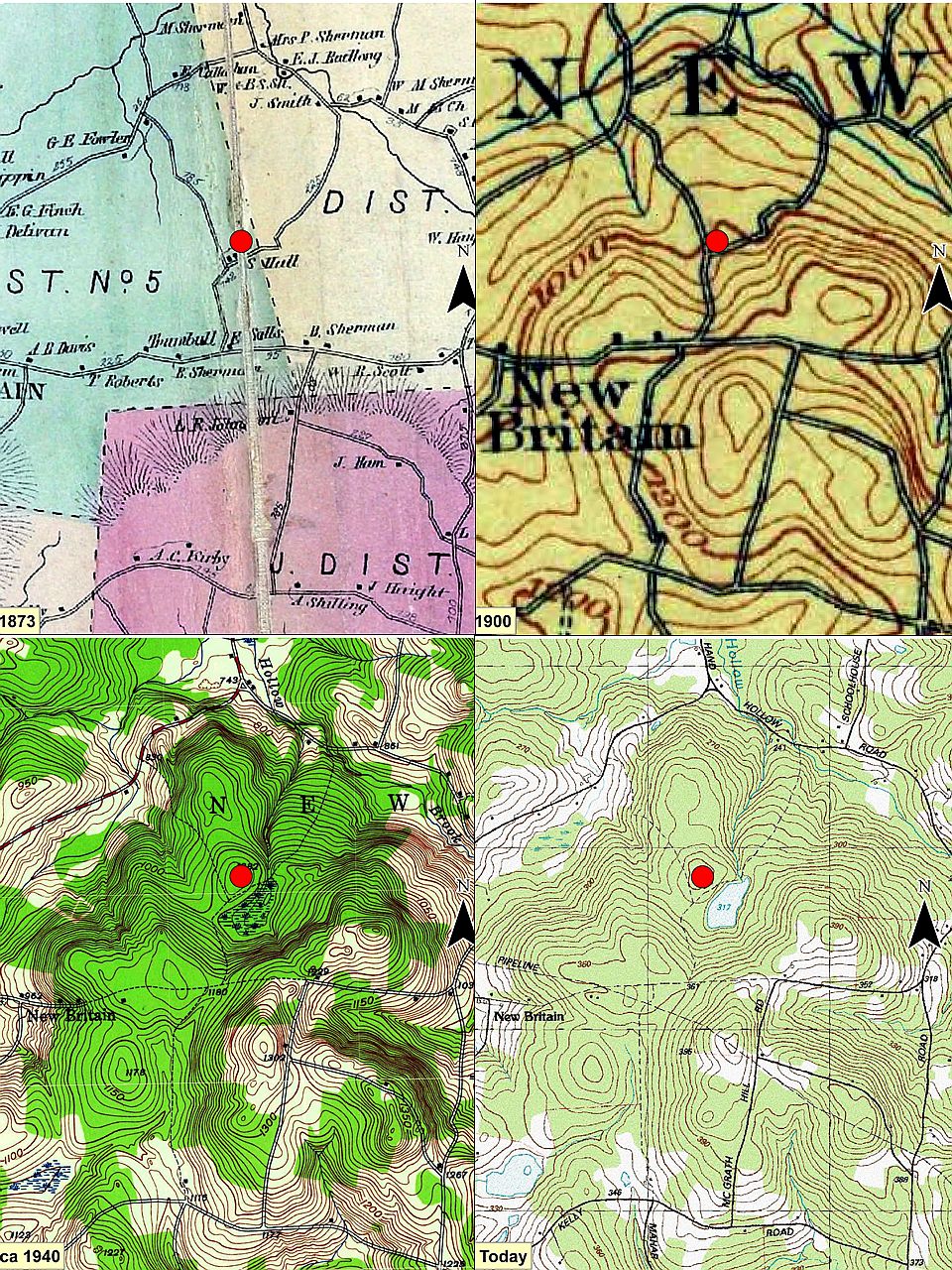 This series of maps show our area starting about 1873 (a map from 1858 is largely similar). The red dot is the house foundation indicated in the second image of this series and located on the north side of what is now the open pond. Notice how the road and some of its connections fade to a dotted shadow and the historical wetland morphs to an open-water pond.
This series of maps show our area starting about 1873 (a map from 1858 is largely similar). The red dot is the house foundation indicated in the second image of this series and located on the north side of what is now the open pond. Notice how the road and some of its connections fade to a dotted shadow and the historical wetland morphs to an open-water pond. This and the next image are historical road pictures from the early 1890s in adjacent Berkshire County (both are from Picturesque Berkshire). They may give one an idea of what this road looked like. This picture shows a relatively well-kept, probably central road. Notice that the low rock ‘wall’ along the road is topped by a rail and cross posts fence, making it high enough to deter at least some animals.
This and the next image are historical road pictures from the early 1890s in adjacent Berkshire County (both are from Picturesque Berkshire). They may give one an idea of what this road looked like. This picture shows a relatively well-kept, probably central road. Notice that the low rock ‘wall’ along the road is topped by a rail and cross posts fence, making it high enough to deter at least some animals. This image may show something a bit closer to what the Hand Hollow road looked like – a somewhat rough and muddy track. The fence along this rock wall has apparently not been maintained.
This image may show something a bit closer to what the Hand Hollow road looked like – a somewhat rough and muddy track. The fence along this rock wall has apparently not been maintained. This photo looks north along the ravine that drains the pond. Much of the late 19th century forest in this area was apparently along this untillable stretch.
This photo looks north along the ravine that drains the pond. Much of the late 19th century forest in this area was apparently along this untillable stretch. The pond itself, with DEC’s handicap-accessible dock and a beaver excluder around the pond drain in the foreground. The pond appears to have been constructed in the 1960s from what was once an extensive wetland. This is a common fate of regional wetlands.
The pond itself, with DEC’s handicap-accessible dock and a beaver excluder around the pond drain in the foreground. The pond appears to have been constructed in the 1960s from what was once an extensive wetland. This is a common fate of regional wetlands.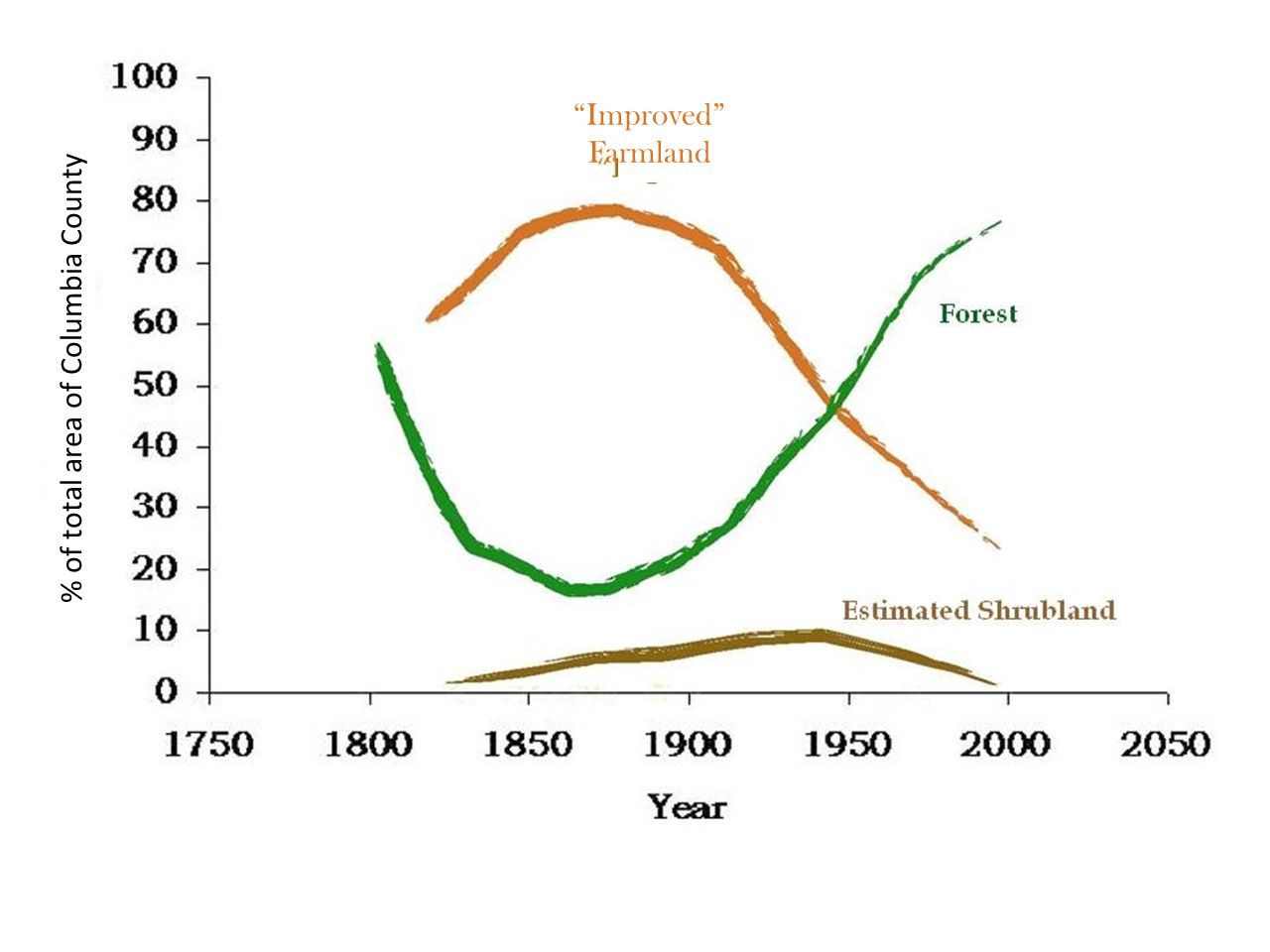 As background for what follows, it helps to quickly review forest history in Columbia County. As is true for much of the Northeast, opening for agriculture was widespread and peaked somewhere around 1875, when 70-80% of the County was open farmland.
As background for what follows, it helps to quickly review forest history in Columbia County. As is true for much of the Northeast, opening for agriculture was widespread and peaked somewhere around 1875, when 70-80% of the County was open farmland.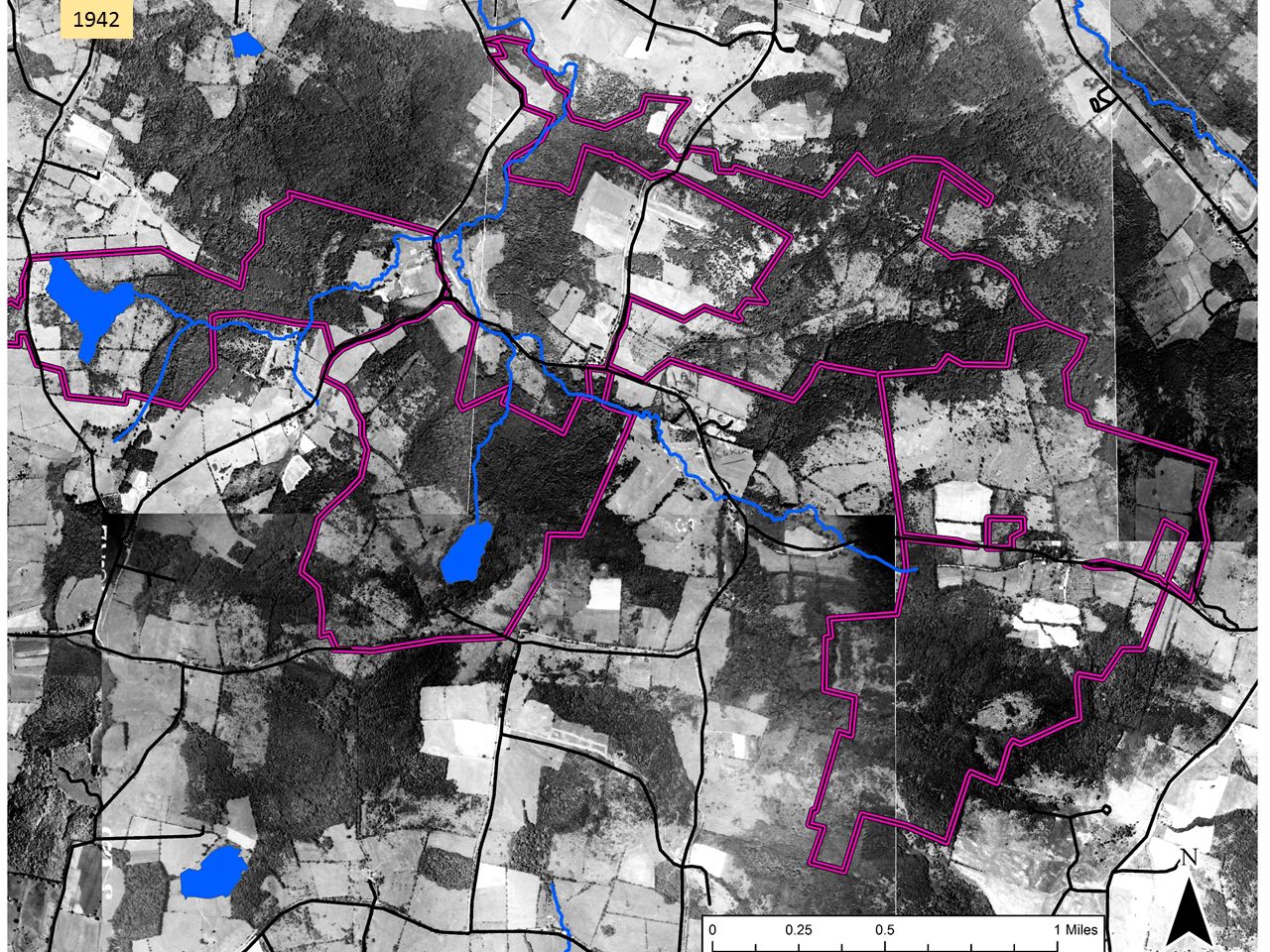 This aerial image from 1942, 50 years or more after maximum clearing, shows a landscape that might be about 50/50 farmland/forest. The purple lines show the approximate extent of the lands shown in the first map.
This aerial image from 1942, 50 years or more after maximum clearing, shows a landscape that might be about 50/50 farmland/forest. The purple lines show the approximate extent of the lands shown in the first map.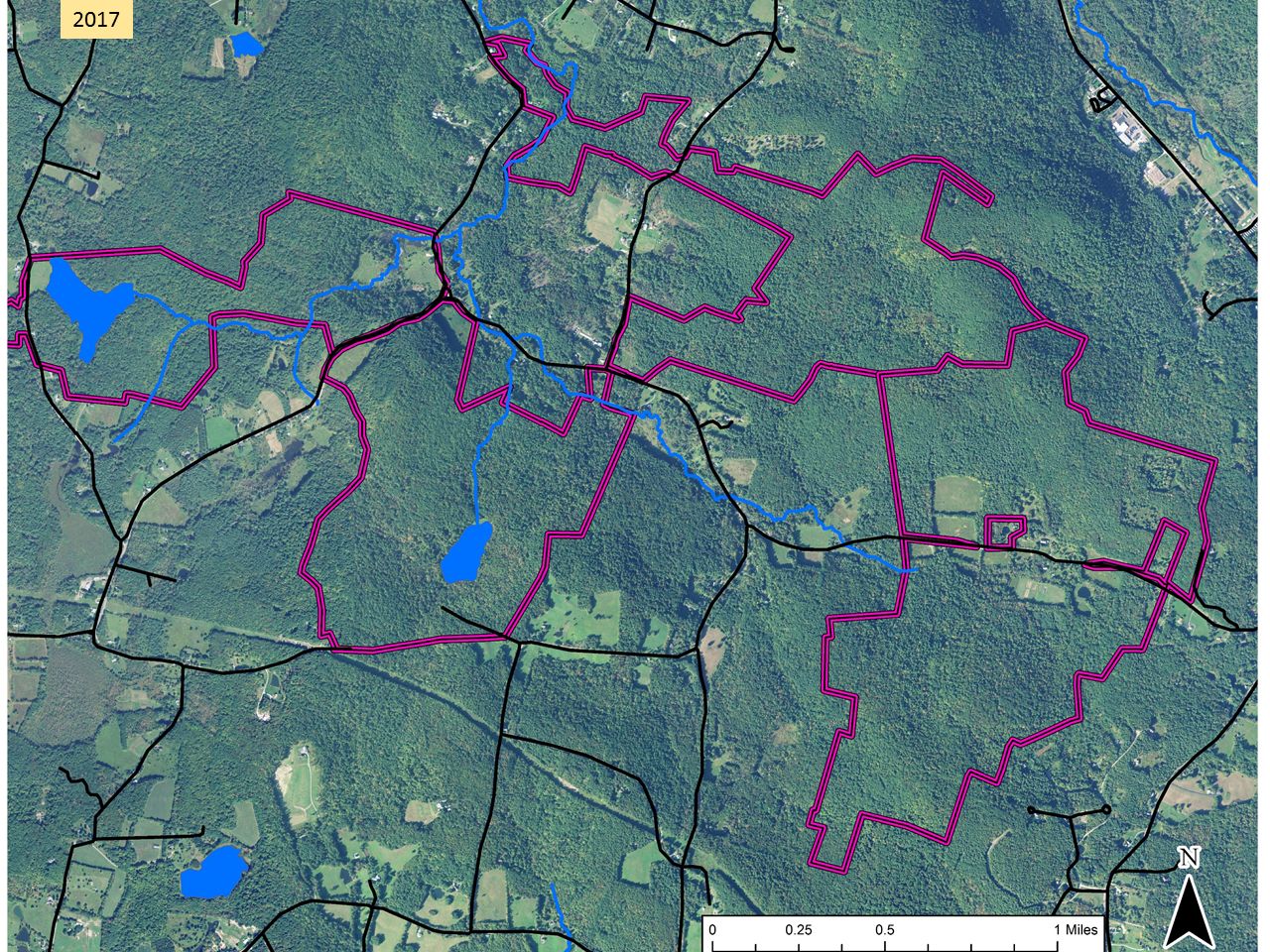 By 2017, farmland was the rare patch in a forest matrix.
By 2017, farmland was the rare patch in a forest matrix.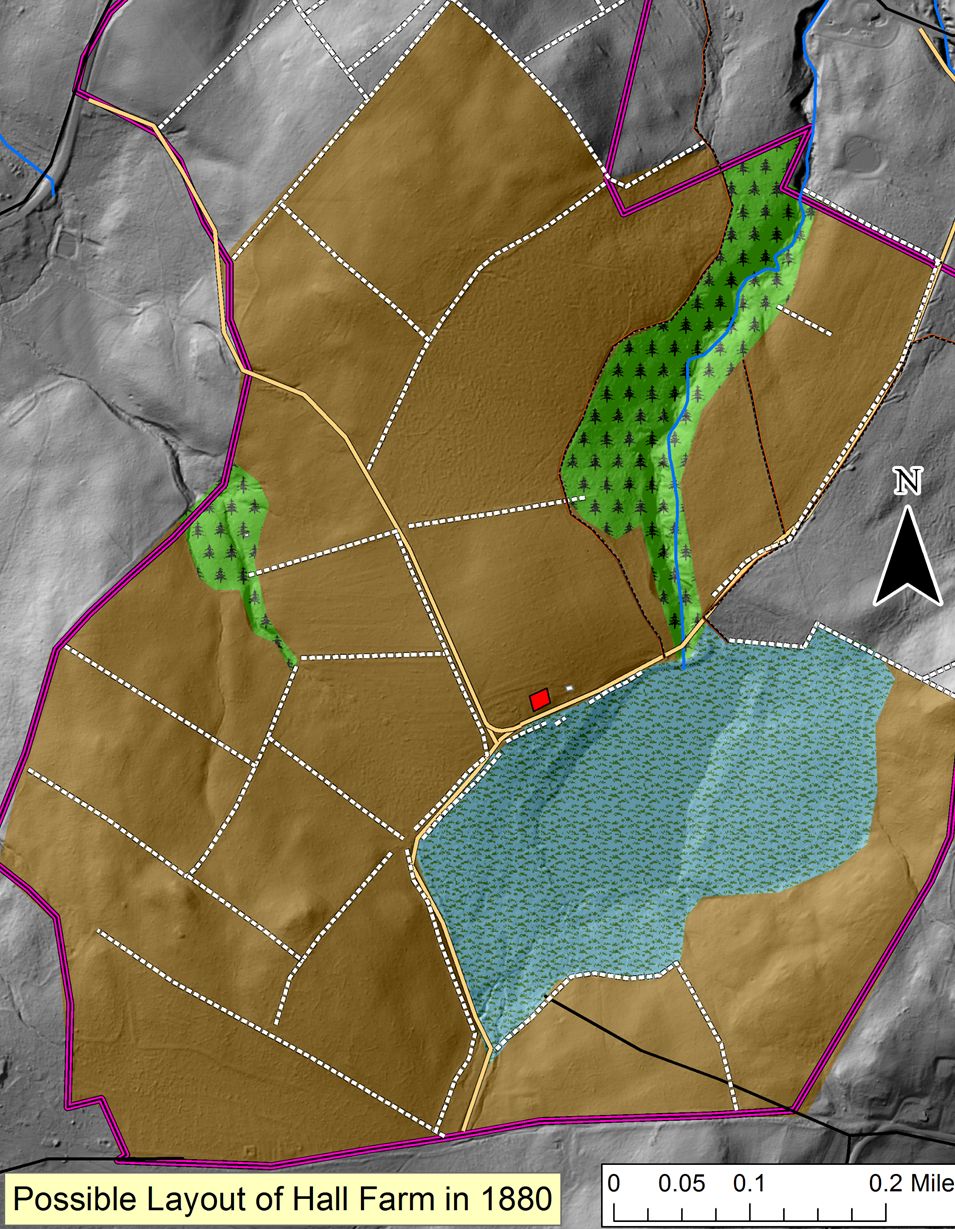 According to an early map, the foundation north of the pond was the home of A. Spencer Hall. Based on census information, walls and guesswork, we have created this sketch of his farm around 1880. This would have been a wide-open landscape composed mostly of fields. The wetland was probably a meadow grazed or hayed as conditions allowed.
According to an early map, the foundation north of the pond was the home of A. Spencer Hall. Based on census information, walls and guesswork, we have created this sketch of his farm around 1880. This would have been a wide-open landscape composed mostly of fields. The wetland was probably a meadow grazed or hayed as conditions allowed. As one approaches the foundation from the northeast, the fact that one is walking through former fields becomes evident: flat ground surface, old-field White Pine and, although it’s somewhat hard to see here, an elevated plow terrace caused by years of plowing almost but not quite to the wall in the foreground.
As one approaches the foundation from the northeast, the fact that one is walking through former fields becomes evident: flat ground surface, old-field White Pine and, although it’s somewhat hard to see here, an elevated plow terrace caused by years of plowing almost but not quite to the wall in the foreground. Just before one arrives at the foundation, one reaches this tangle of Honeysuckle – the most evident reminder that this was once somebody’s backyard.
Just before one arrives at the foundation, one reaches this tangle of Honeysuckle – the most evident reminder that this was once somebody’s backyard. This is a sizable foundation with a stone-lined cellar. In the far corner, is a pile of bricks (a chimney?) while in the near corner a well hole seems to be attached to the house. Somebody familiar with the property told us that these and other bricks were made on-site. Judging by aerial imagery, the house probably disappeared in the third quarter of the 20th century.
This is a sizable foundation with a stone-lined cellar. In the far corner, is a pile of bricks (a chimney?) while in the near corner a well hole seems to be attached to the house. Somebody familiar with the property told us that these and other bricks were made on-site. Judging by aerial imagery, the house probably disappeared in the third quarter of the 20th century. Just beyond the house is the crossroads where the two arms of the public road’s ‘Y’ meet. This photo looks northwest along the western arm.
Just beyond the house is the crossroads where the two arms of the public road’s ‘Y’ meet. This photo looks northwest along the western arm. Below the house, a ca. 5’ high stone bank supports the road, presumably helping to prevent it from eroding into the wetland. In this picture, one’s back is to the pond, and the road runs along the ground which is even with the top of the rocks.
Below the house, a ca. 5’ high stone bank supports the road, presumably helping to prevent it from eroding into the wetland. In this picture, one’s back is to the pond, and the road runs along the ground which is even with the top of the rocks.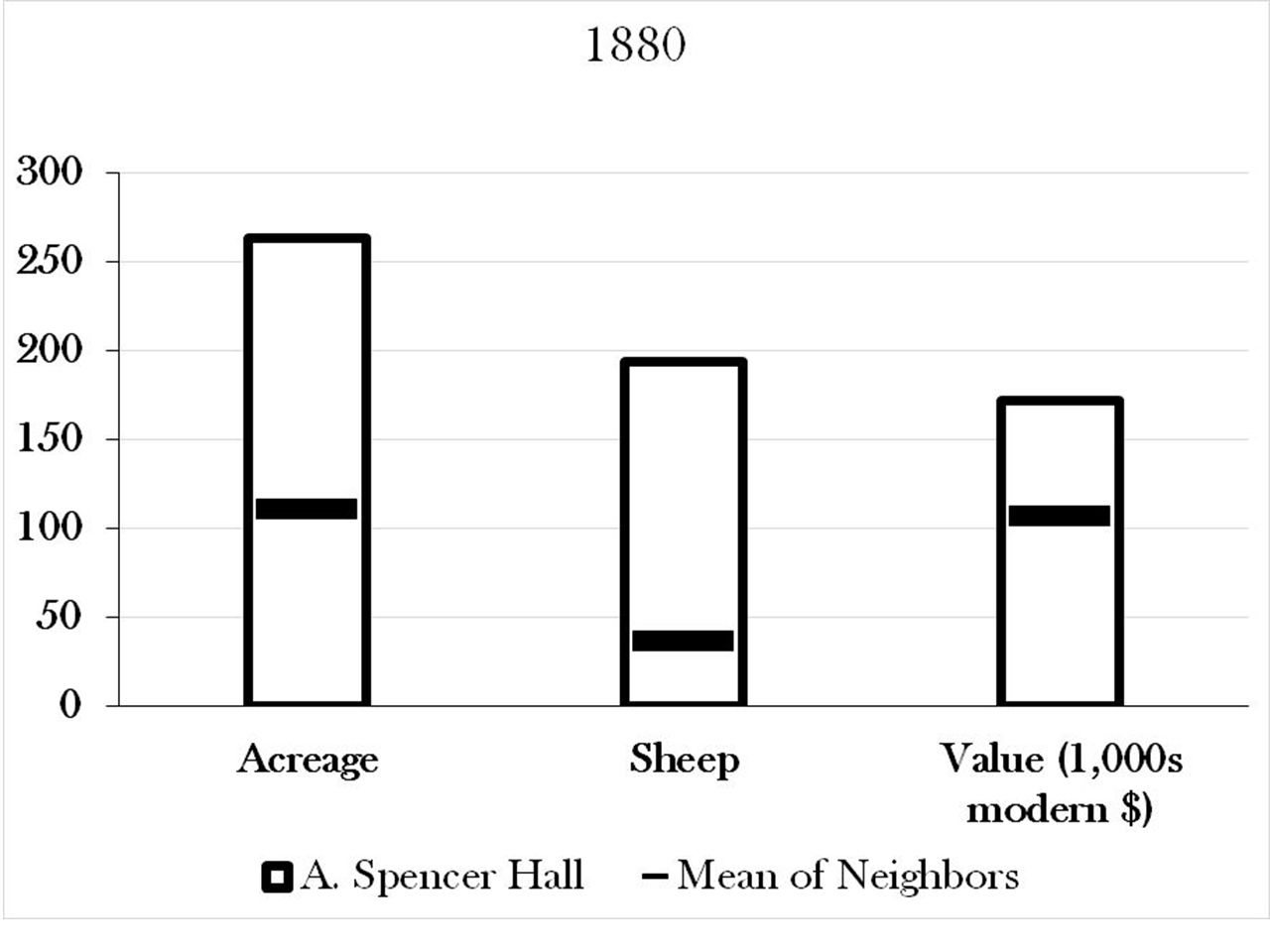 Spencer Hall’s farm was, according to the census records, not a particularly small or poor one. It had twice the average acreage of its neighbors, nearly ten times the number of sheep, and was valued about 40% above that of its average neighbor (at ca. $172,000 in modern currency).
Spencer Hall’s farm was, according to the census records, not a particularly small or poor one. It had twice the average acreage of its neighbors, nearly ten times the number of sheep, and was valued about 40% above that of its average neighbor (at ca. $172,000 in modern currency).













 Pictures of Roads from Picturesque Berkshire, 1893. Most 19th century photographs are of people or single buildings rather than landscapes. While this book contains its fair share of those, it also includes many photos of roadsides and fields. The landscape is late-19th century with wire beginning to replace rock walls and split rail fences, but the landscape still contains many earlier traces, and the photographs of roadsides on this page, although they do not clearly show Goldenrods, likely show where you could have found them. Note that both hayfield and pasture are rougher lands than their current incarnations and probably left more space for diversity.
Pictures of Roads from Picturesque Berkshire, 1893. Most 19th century photographs are of people or single buildings rather than landscapes. While this book contains its fair share of those, it also includes many photos of roadsides and fields. The landscape is late-19th century with wire beginning to replace rock walls and split rail fences, but the landscape still contains many earlier traces, and the photographs of roadsides on this page, although they do not clearly show Goldenrods, likely show where you could have found them. Note that both hayfield and pasture are rougher lands than their current incarnations and probably left more space for diversity.






































 n reads, “These snow crystals are the product of a very great storm, and they travelled a long distance before reaching the earth. They were generated in a very high frigid altitude. When these singular snow crystals descended they fell in parachute fashion, the larger section downward.” How Bentley and Thompson knew the details of this biography is unclear, but whether or not they were 100% correct, Bentley’s observations and the ‘thought experiments’ that attempted to make sense of them opened up a new world, and Thompson realized and expressed its poetry.
n reads, “These snow crystals are the product of a very great storm, and they travelled a long distance before reaching the earth. They were generated in a very high frigid altitude. When these singular snow crystals descended they fell in parachute fashion, the larger section downward.” How Bentley and Thompson knew the details of this biography is unclear, but whether or not they were 100% correct, Bentley’s observations and the ‘thought experiments’ that attempted to make sense of them opened up a new world, and Thompson realized and expressed its poetry. the water molecule itself determines the shape of the initial base (a hexagonal plate), but the pattern of any individual crystal’s growth is the product of temperature, humidity and history. If the air is very dry and/or cold, for instance, the snowflake will likely fall to earth as a hexagonal plate or tube. If, on the other hand, the air is humid and only moderately cold, each of the six corners will begins to sprout a feather-like arm. As a flake travels through the clouds, it may well enter different temperature and humidity zones, each of which will cause the crystal to grow in new ways. Thawing and re-freezing may occur, and ‘rough seas’ may break flakes or glue them together. The huge diversity of snowflakes occurs because it is very unlikely that any two flakes will share exactly the same biographies during their trip to the ground.
the water molecule itself determines the shape of the initial base (a hexagonal plate), but the pattern of any individual crystal’s growth is the product of temperature, humidity and history. If the air is very dry and/or cold, for instance, the snowflake will likely fall to earth as a hexagonal plate or tube. If, on the other hand, the air is humid and only moderately cold, each of the six corners will begins to sprout a feather-like arm. As a flake travels through the clouds, it may well enter different temperature and humidity zones, each of which will cause the crystal to grow in new ways. Thawing and re-freezing may occur, and ‘rough seas’ may break flakes or glue them together. The huge diversity of snowflakes occurs because it is very unlikely that any two flakes will share exactly the same biographies during their trip to the ground.
